After extensive preparations and long-lasting efforts used on a project managed by Matija Kropek, M.Pharm., a new stage in Belupo's life has successfully started
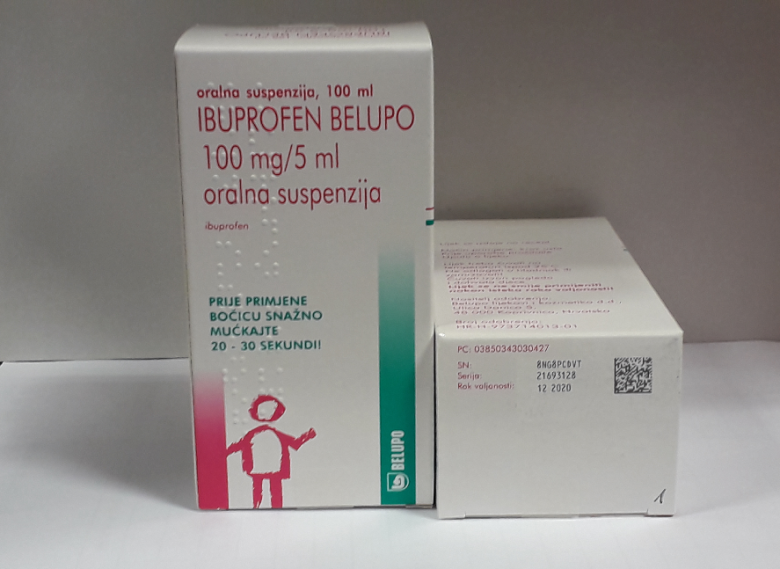
After extensive preparations and long-lasting efforts used on a project managed by Matija Kropek, M.Pharm., a new stage in Belupo's life has successfully started: serialization of prescription medicines to comply with the requirements of Directive (EU) 2011/62 and Commission Delegated Regulation (EU) 2016/161 on the protection of medicines against falsification. From now on, each pack of a prescription medicine intended for any EU market will have a unique code and other prescribed security features to reduce the possibility of falsifying medicines and thus increase the safety of its use. Belupo will use serialization on all its packaging lines and will send data about serialized batches to the EU Hub, from where they will be forwarded to appropriate national systems.
